
Your Trusted Partner in Contamination Control
As industries demand higher standards in contamination control and environmental monitoring, Link Lab continues to evolve and expand. As a Gold Channel Partner of TSI Inc., a leader in contamination control, we bring cutting-edge solutions to a variety of high-tech sectors.
Expanding Across Industries
We proudly serve industries including aerospace, space, defense, semiconductor, and battery manufacturing while maintaining our strong foundation in pharmaceutical, healthcare, and life sciences—the cornerstone of our reputation for excellence.
Advanced Contamination Control Solutions
At Link Lab, we provide state-of-the-art technology to maintain contamination-controlled environments:
- Airborne Particle Counters & Air Samplers – Precision monitoring for cleanroom compliance.
- Custom Cleanroom Monitoring Systems – Real-time contamination tracking with scalable solutions.
- Advanced Alarm and Safety Systems – Instant alerts for critical contamination events.
Comprehensive Support & Expertise
We go beyond technology by offering installation, calibration, qualification, technical expertise, and customer support, ensuring seamless integration and optimal performance.
Unmatched Experience & Accreditation
With over 20 years of experience in environmental monitoring and ESYD ISO17025 accreditation for laboratory temperature and humidity calibration, Link Lab is recognized for its outstanding credentials and commitment to excellence.
Partner With Us
We understand that every client’s needs are unique. That’s why we collaborate closely to develop tailored solutions that deliver superior performance, value, reliability, and longevity.
Contact us today to discover how Link Lab can support your contamination control and environmental monitoring needs.
AeroTrak®+ A100 Series Particle Counter
Ready for Measurement.
Engineered for you to make your job easy.
AeroTrak™+ Portable Airborne Particle Counters (APCs) are ideal for all industries for cleanroom certification, monitoring and specialized tasks including gas testing and filter scanning.

Flow Rate 1 CFM (28.3 LPM) – 3.5 CFM (100 LPM) / Channel Sizes 0.3 µm to 10 µm,(6) user selectable channels.
Their small size, flexible power options and wireless capabilities allow installation where space is limited. Worry-free monitoring with best-in-class data buffering reduces risk of data loss during network outages. Only the AeroTrak™+ Remote Particle Counters are backed by an industry exclusive 5 year laser warranty.
All TSI AeroTrak+ Remote Particles Counters and AeroTrak Remote Particle Counters comply with ISO 21501-4.
TSI Remote Particle Counters are available in a variety of:

-
- Flow rates
- Particle sizes
- Analog output options
- Wireless enabled
- VHP resistant remote particle counter models available
- 5 years Laser Warranty
AeroTrak-Plus Remote Particle Counter 7000 Series

Flow Rate 0.1 CFM (2.83 L/min), 1 CFM (28.3 L/min) / Channel Sizes 0.2 to 25 μm
AeroTrak-Plus Remote Particle Counter with Integrated Pump 6000 Series

Flow Rate 0.1 CFM (2.83 L/min) / Channel Sizes 0.2 to 25 μm / Integrated Pump
AeroTrak Remote Particle Counter with Integrated Pump 6510 & 6510-VHP

Flow Rate 1 CFM (28.3 L/min) / Particle Size Range 0.5 to 25 μm / Integrated Pump / VHP Option
AeroTrak Remote Particle Counter with Integrated Pump 6310

Flow Rate 1 CFM (28.3 L/min) / Particle Size Range 0.3 to 25 μm / Integrated Pump
AeroTrak Remote Particle Counter 7110

Flow Rate 1.0 CFM (28.3 L/min) / Particle Size Range 0.100 to 10.0 µm
AeroTrak Cleanroom Condensation Particle Counter 9001-CPC
The AeroTrak 9001 Condensing Particle Counter for Controlled Environments is a one-of-a-kind instrument, specifically designed for electronic industry to ensure the safety required for monitoring ISO 1 and ISO 2 clean rooms, helping to protect both products and the production process from potential damage.

10nm to 100nm size range ,0.1 CFM (2.83 L/min) flow rate , Ultra-low false count rate
The AeroTrak® TSI 9110 portable particle counter offers precise particle measurement down to 0.100 μm!

Flow Rate 1 CFM (28.3 L/min) ±5% accuracy / Particle Size Range 0.100 to 10.0 µm
Being lightweight and easy to carry, they are well-suited for cost-effective spot checking. It is the ideal way to:
- Pinpoint sources of contamination
- Test filters
- Perform indoor air quality (IAQ) investigations
All TSI AeroTrak™ Handheld Particle Counters comply with ISO 21501-4. AeroTrak Handheld Particle Counter Model 9306 measures up to six channels of simultaneous data; Model 9303 monitors up to three channels.
AeroTrak Handheld Particle Counter 9306

Flow Rate 0.1 CFM (2.83 L/min) ±5% accuracy / Particle Size Range 0.3 to 25 µm
AeroTrak Handheld Particle Counter 9303

Flow Rate 0.1 CFM (2.83 L/min) / Particle Size Range 0.3 to 25 µm
AeroTrak+ 7010 Active Air Sampler (AAS)

The TSI AeroTrak™+ 7010 provides reliable and accurate microbial monitoring for aseptic manufacturers in pharmaceutical Grade A and B environments, utilizing external vacuum systems. Its active flow measurement and proactive flow alarms empower cleanroom technicians to address facility conditions promptly, minimizing production waste.
Seamlessly integrating with TSI Facility Monitoring Software, the active air sampler ensures uninterrupted data availability, keeping critical information at your fingertips for confident decision-making.
BioTrak Real-Time Viable Particle Counter
The only accepted method to replace active and passive air sampling in Grade A
This biofluorescent particle counter provides real-time counts of total and viable particles in pharmaceutical manufacturing environments to reduce aseptic interventions, improve root-cause investigations, and increase process knowledge
Meeting Requirements for EU GMP Annex 1 — Features and Benefits

Introducing the BIOTRAK® Real-Time Viable Particle Counter
Best-in-Class Technology: A cutting-edge solution in real-time airborne viable particle detection.
Real-Time Detection: Simultaneously measures total and viable particle counts using TSI’s patented Laser Induced Fluorescence (LIF) technology.
All-in-One Instrument: Combines viable particle detection, total particulate measurement, and integrated particle collection in a portable design.
Key Applications:
- Continuous Monitoring of aseptic environments
- Enhanced Investigations of root cause analysis
- Minimize Risks and expedite room release
- Advanced Detection Capabilities in Real-time of airborne viable particles
- Concurrent measurement of total and viable particles.
- Flexible Integration as standalone or as remote with FMS for automated monitoring.
- Simplified Sampling , requires only a single isokinetic probe at the sample site.
We detect all particles
Major industry publication features — click here to see what your industry peers are publishing, including but not limited to:
PDA Journal of Pharmaceutical Science and Technology — the primary source of peer-reviewed scientific and technical papers on topics related to pharmaceutical/biopharmaceutical manufacturing, sterile product production, aseptic processing, pharmaceutical microbiology, quality, packaging science, and other topics relevant to PDA members
PEMM (Process and Environmental Monitoring Methods) Group — a collaborative group comprised of leading pharma industry experts
BioPhorum — a business-to-business membership organization consisting of ten phorums leading more than 110 industry-changing initiatives with the help of 7,500 active subject matter experts
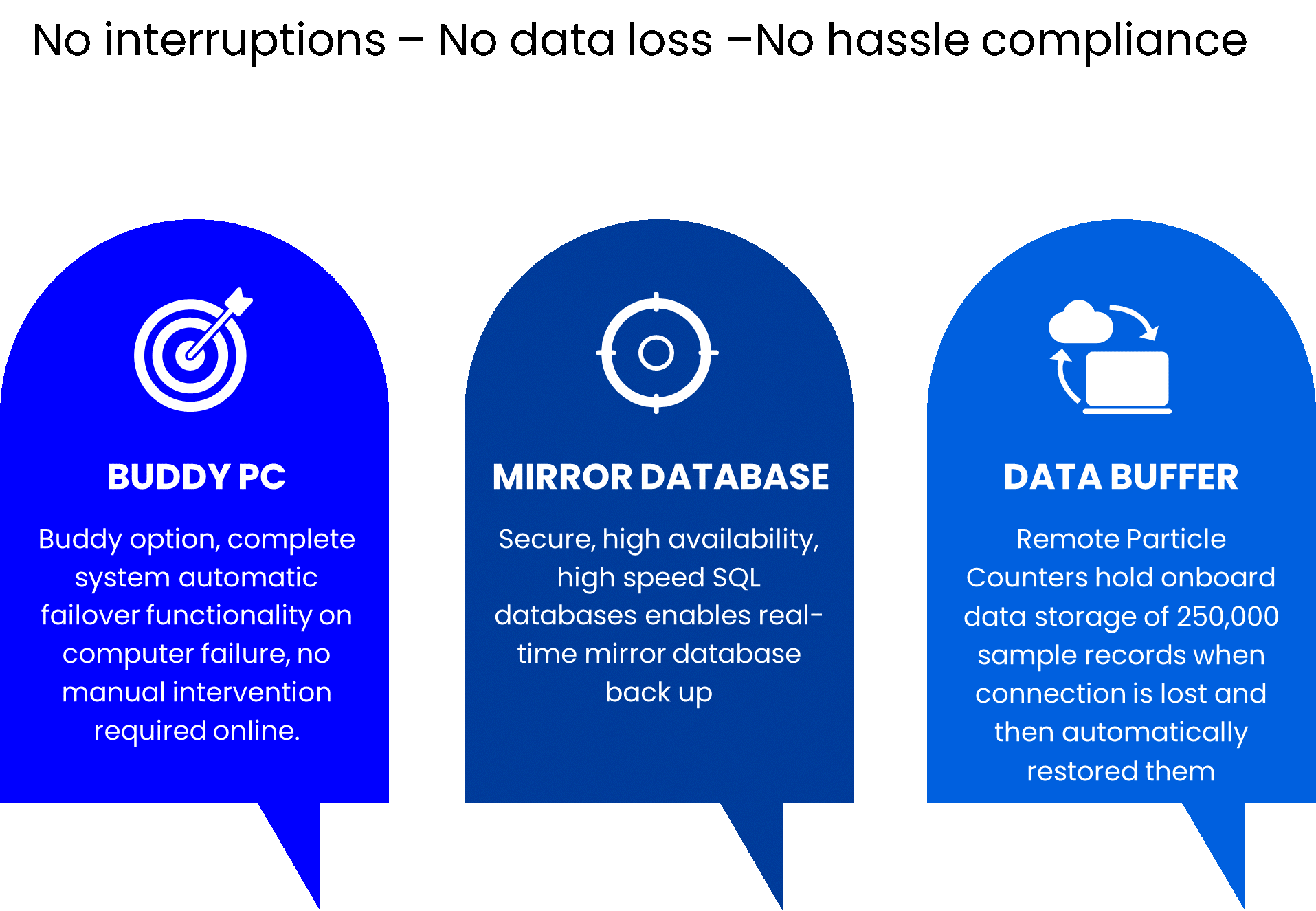
Why to invest on Biotrak
- Fully automated process ( no interruption 24/7)
- EU GMP Annex 1 Compliant
- Increase of aseptic production up to 25%
- Time and cost saving (no microbial consumambles, incubations)
- Product always protected
- Return on investment – Contact us for an ROI simulation on your production
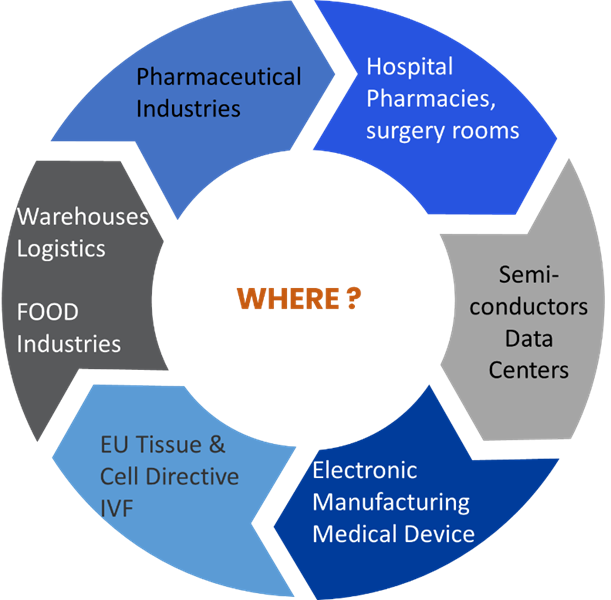
Why and Where FMS ?
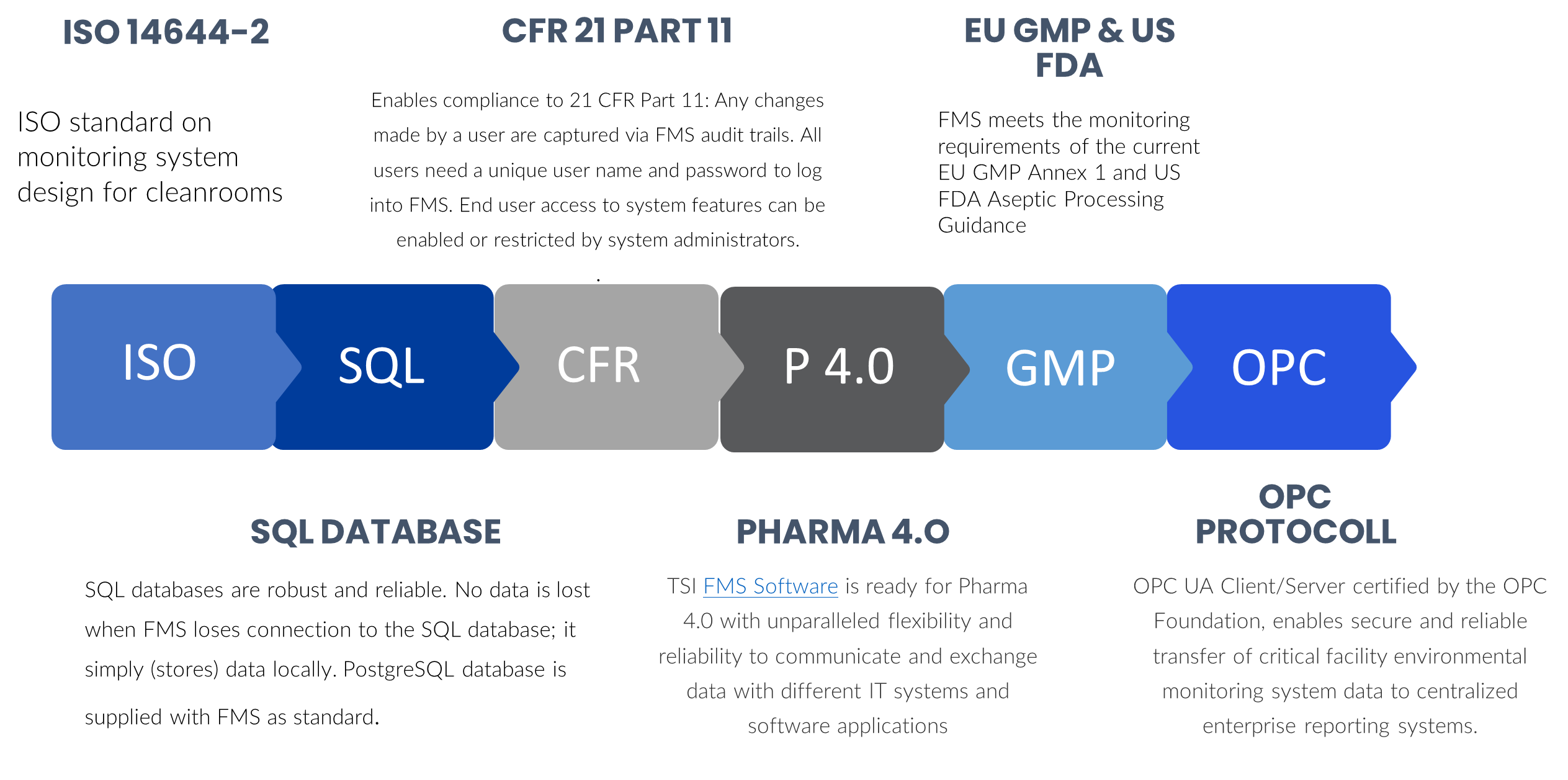
Complies With
Compliance with all International Standards and Protocols

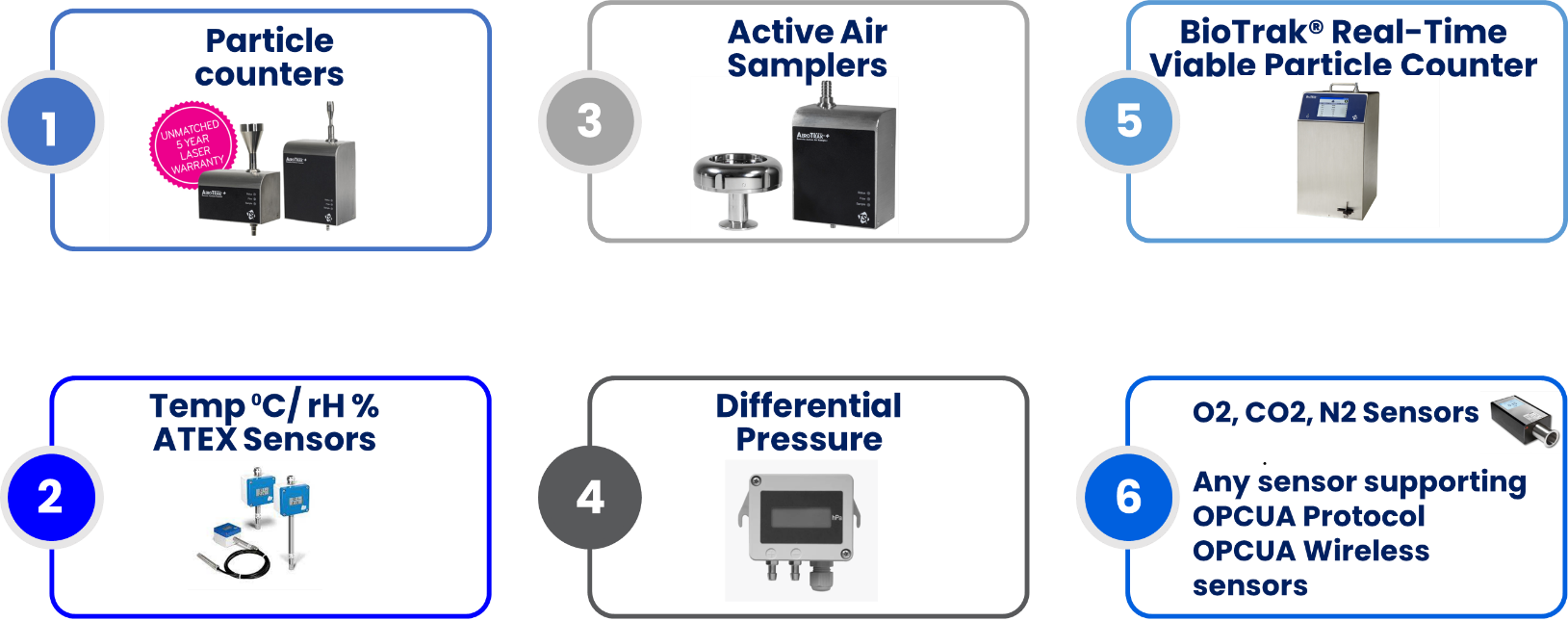
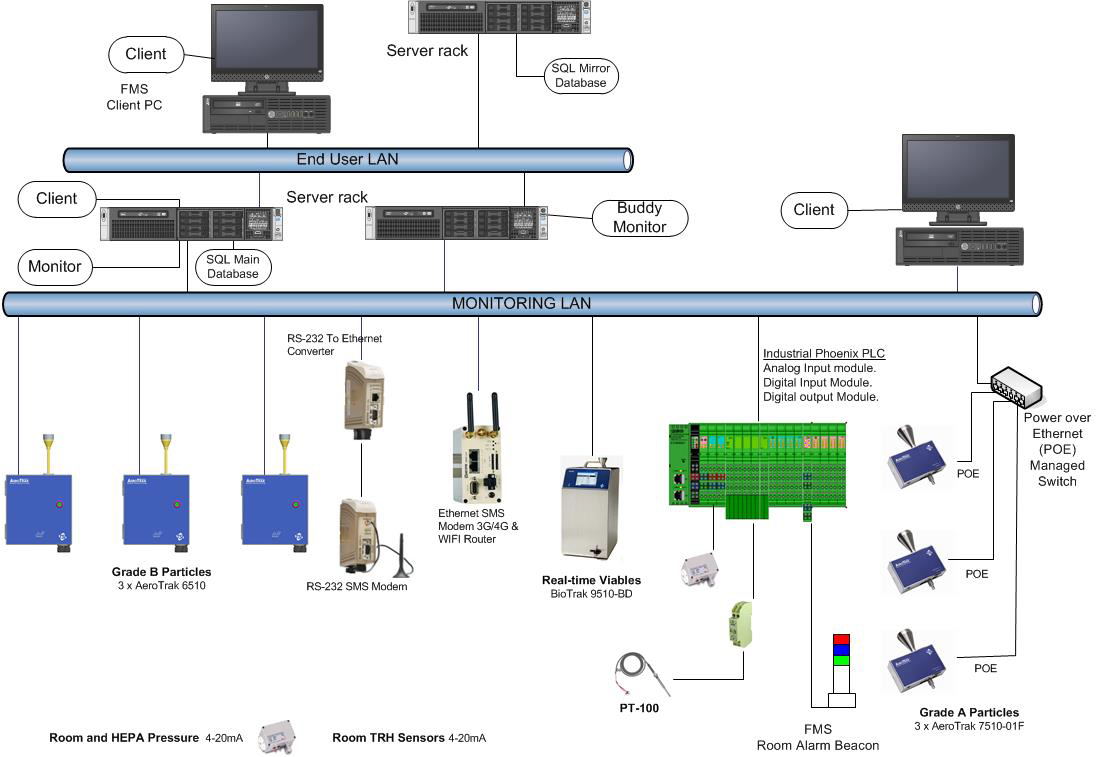
List of Sensors in FMS
Timeline of FMS System
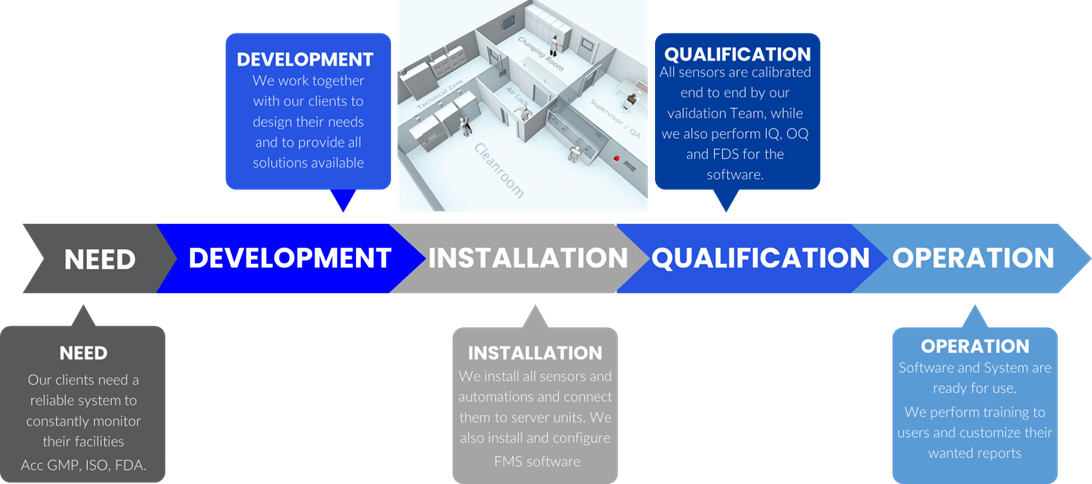
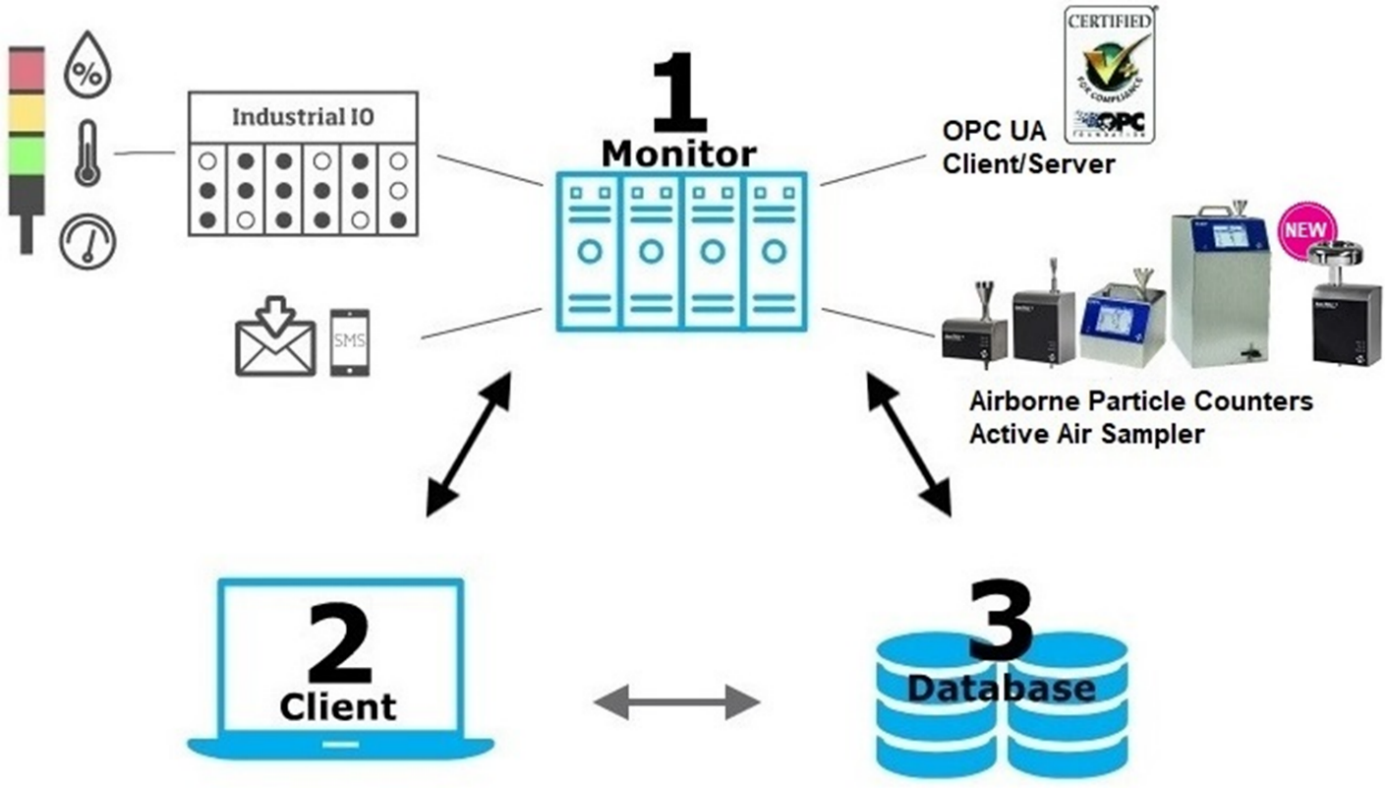
FMS Overview
Alarms




Safety of data
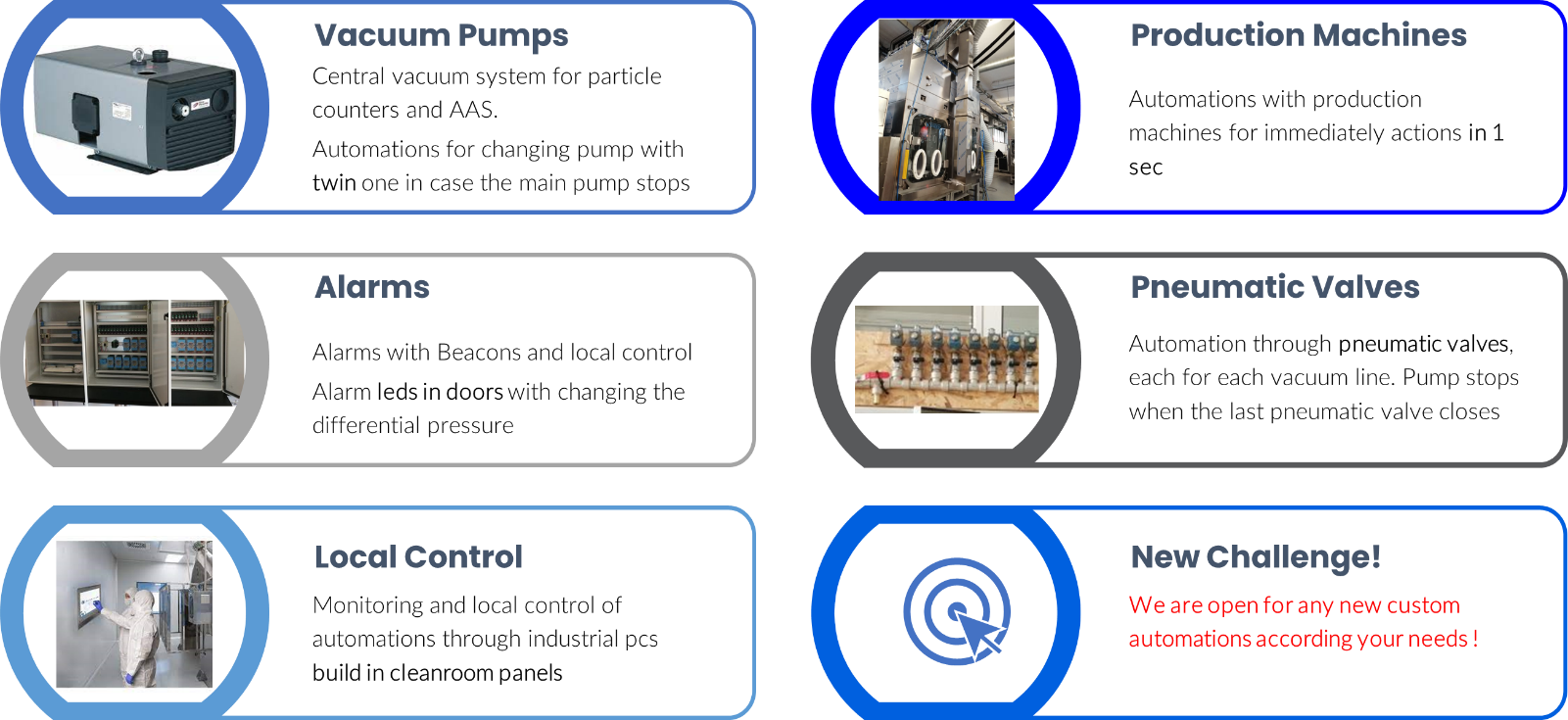
Automations
Our excellence in automations makes the difference
The Life Sciences and Pharma Division at TSI, Inc. comprises a diverse team of professionals dedicated to serving the regulated Life Sciences industries. This team includes experts in regulations and applications who provide comprehensive support and training to customers worldwide for both viable and nonviable monitoring.
TSI particle counters have enhanced data integrity capabilities. This is most critical for pharmaceutical, medical device and life science manufacturing industries to ensure complete, consistent, and accurate data according to ALCOA+ principles and FDA CFR Part 11 regulatory requirements.
TSI particle counting technology is used by national and standards labs worldwide. Rely on our experience to confidently ensure regulatory compliance and improve quality.
For confident, reliable, no-hassle compliance and data integrity, CLICK HERE to learn more about TSI Complete Facility Monitoring Systems.
Contaminated products pose an obvious health risk to patients. That is why pharmaceutical/life science companies are required to manufacture in controlled environments as part of their overall contamination control program. These environments must be monitored to assure control is maintained. This makes it critical that the monitoring instruments and software implemented must be capable of generating reliable, timely results with demonstrable data integrity.
TSI: Reliable, Timely Results with Secure Data Integrity
TSI offers environmental monitoring systems that integrate total particle counting, viable particle monitoring (active air sampling) as well as temperature, pressure and humidity. Everything you need to verify appropriate environmental conditions for your aseptic process.
TSI AeroTrak®+ Remote Particle Counters can buffer over half a year of one minute continuous particle sample results. When coupled with the reliable TSI FMS Software, no other system does more to prevent loss of data.
When working with high value products, waiting for one minute to get a particle count may be too long. TSI AeroTrak+ Remote Particle Counters can monitor on a per second basis to alarm the instant an excursion occurs.
TSI VHP resistant AeroTrak+ Remote Particle Counters and VHP resistant AeroTrak Remote Particle Counters with Integrated Pumps are available for worry-free use in isolators or other environments that employ vaporized hydrogen peroxide for sanitization.
TSI AeroTrak+ Remote Particle Counters are designed for reliability and backed by a best-in-class 5 year laser warranty
TSI offers system integrated AeroTrak+ Remote Active Air Sampler specifically designed for aseptic manufacturing lines. To go beyond compendial methods, the BioTrak® Real-Time Viable Particle Counter (a biofluorescent particle detector that meets EU GMP Annex 1 requirements) offers continuous sampling without interventions. An alternative and rapid microbial testing method (ARMM) for viable air testing, it performs ISO compliant total particle counting and real-time viable particle detection.
With best-in-class features, such as OPC UA Client/Server, distributed databases, and hot standby, TSI FMS Software is ready for Pharma 4.0 with unparalleled flexibility and reliability to communicate and exchange data with different IT systems and software applications.
Now available and ready for measurement. AeroTrak+ Portable APCS are engineered for you to make your job easy.All TSI AeroTrak Portable Particle Counters are designed to simplify the cleanroom classification process per common standards including EU GMP Annex 1 and ISO 14644-1. This is done by either using the instrument’s built-in reporting capabilities or utilizing the included TrakPro™ Lite Secure Software to generate high quality reports.
High flow rate instruments are available for rapid sampling of a full cubic meter of air.
TSI AeroTrak Portable Particle Counters and BioTrak Real-Time Viable Particle Counter [using biofluorescent particle counting (BFPC) technology] can operate with the included TrakPro Lite Secure Software in an optional mode that is specifically designed to meet ALCOA+ data integrity principles.
TSI FMS Software (with OPC UA Client/Server functionality) can also be seamlessly integrated with AeroTrak Portable Particle Counters and BioTrak Real-Time Viable Particle Counter, as well as AeroTrak+ Remote Particle Counters, AeroTrak+ Remote Active Air Sampler, and environmental sensors, to centralize all particle counting data and to continuously monitor cleanrooms and isolators.

Hospital Room Pressure Monitors and Controls
Hospital room pressure monitors and controls help keep patients and staff safe. TSI’s quality products use proven technology and engineering for the best possible health, safety, and compliance outcomes. Hospitals around the world choose TSI room pressure products for these features and benefits: 
- Most accurate measurements of room pressure differential in the industry, using industry-leading through-the-wall (TTW) sensor technology
- Extremely stable performance with virtually no need to recalibrate sensors
- Simple, intuitive displays easily understood by staff
- Native BACnet allows seamless communication with Building Automation System (BAS)
Top choice room pressure monitors and controls
TSI designed PresSura™ Room Pressure Monitors (integrated with TTW sensors) and Controls to help healthcare organizations meet requirements. They enable compliance with standards, including:
- ASHRAE 170 Ventilation in Healthcare Facilities
- U.S. Centers for Disease Control HICPAC (Healthcare Infection Control Practices Advisory Committee)
- CSA Z317.2-15 Special Requirements for Heating, Ventilation and Air Conditioning (HVAC) Systems in Healthcare Facilities
- Other medical, pharmaceutical, and construction standards
PresSura Monitors and Controls are the system of choice for hospital room pressure monitoring. For over 30 years, hospitals, laboratories, and cleanroom facilities have chosen TSI products for monitoring of their critical environments. TSI products are online in isolation rooms, protective environments, operating rooms, and other controlled areas. PresSura Room Pressure Monitors and Controls are also extremely effective for laboratory monitoring and cleanroom monitoring. By accurately measuring room pressure differential, ventilation rates (air changes per hour), temperature, and humidity, TSI products control the spread of contaminants in applications that matter.
PresSura Hospital Room Pressure Monitors RPM10

Monitors pressure in hospital rooms (maximum 1 room).
PresSura Hospital Room Pressure Monitors RPM20

Monitors pressure, ventilation, temp and humidity in hospital rooms (maximum 3 rooms).
PresSura Room Pressure Monitors RPM20-CC

Monitors key environmental parameters for compounding pharmacies and other cleanrooms.
PresSura Hospital Room Pressure Controllers RPC30

Controls pressure, temp, humidity and ventilation in hospital rooms (maximum 1 room).
Speedy Glove
Portable, Automatic Glove Integrity Tester
Speedy Glove is a battery-operated device designed for leak testing gloves used on isolators or RABS, ensuring a strict barrier between the isolated environment and external surroundings. It conducts pressure decay integrity tests in compliance with ISO 14644-7 Annex E.5 requirements.

Main Features
- Compact size
- Extremely fast operation
- Highest accuracy
- Complete touch-screen
- RFID technology
- Wi-Fi ready
- VPHP compatibility for Reverse Test
- cGMP compliance
Speedy Glove: Versatile Glove Integrity Tester for All Isolators
Speedy Glove is compatible with all isolators equipped with glove flanges, making it suitable for applications such as aseptic processes, containment processes, handling APIs and HAPIs, and working with radiopharmaceuticals. It supports all glove flanges currently available on the market.
Unique Features and Patented Technology
Speedy Glove stands out in performance and flexibility due to its innovative design. A patented rapid inflation system fills the glove with air in just seconds, reducing testing time by 3–5 minutes (approximately 30% faster than similar devices). Unlike other systems, it operates independently without needing pneumatic circuits or compressed air tanks, managing the entire glove integrity testing process autonomously.
Link Lab Ltd is committed to delivering top-quality services, supported by our fully equipped facility in Athens. This enables us to effectively serve customers across Greece, Cyprus, and neighboring countries.
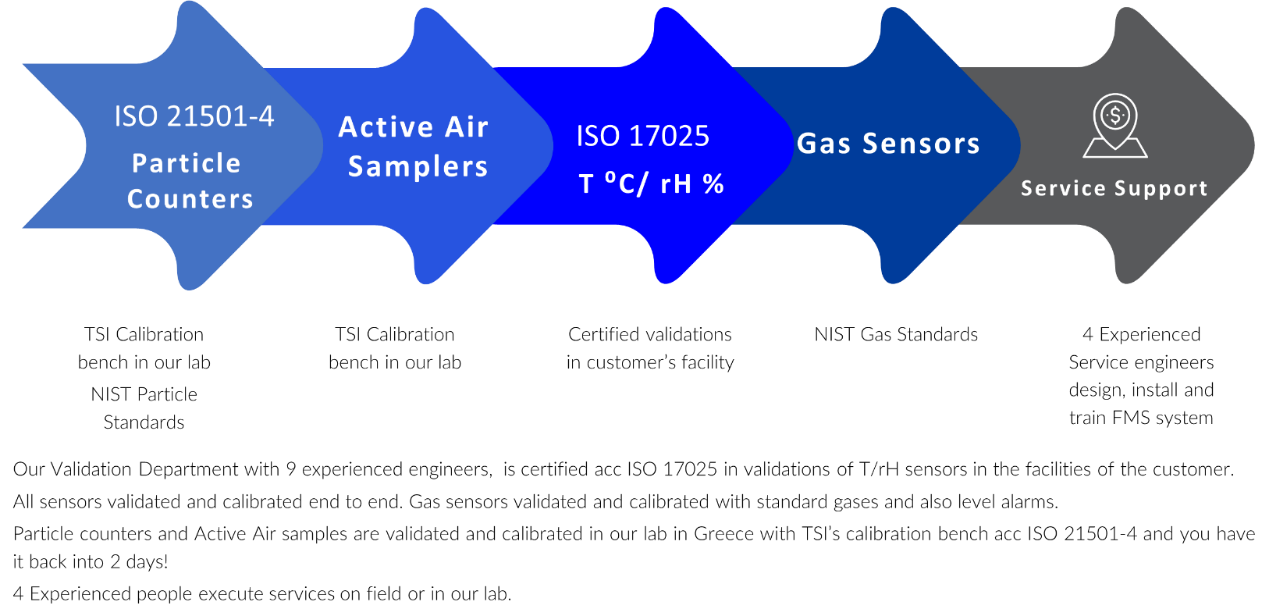
Aerosol particle counters are calibrated in strict accordance with ISO 21501-4:2018 standards. This process includes verifying and adjusting particle sizing, resolution, counting efficiency, flow rate, signal ratios, and zero count. Each calibration is carried out using NIST-traceable standards, and a certification is provided for every completed calibration.
Our services are as follows:
- In-House Particle Counter Calibrations (portable, remote, handheld)
- Particle Counter Calibration to ISO 21501-4 (on compliant particle counters)
- In-House calibration on Air Samplers (portable and remote)
- In-House calibration of anemometers, portable thermo-hydrometers, micromanometers, high pressure diffusers,
- On site calibration of differential manometers, temperature and humidity sensors according ISO 17025
- Traceable reference units for Aerosol particle counters
Particle Counter Calibration includes:
- Particle counter adjustments back to factory standards
- Certificate of calibration issued for each instrument
- Preventative maintenance
- Instrument condition (as found and as left data available)
- NIST traceable standards
Key Benefits of Direct TSI Particle Counter Service and support
- Service Staff are certified to support all revision levels of your TSI products
- Access to the most current software/firmware updates for your TSI products
- Direct Remote Service Support for your TSI hardware and software issues
- Dedicated escalation process for resolving unique issues with your TSI products
- Robust root cause analysis and documentation to support your audit requirements
Calibration of sensors FMS
Providing Validation Excellence
Commitment to Quality
At Link Lab Ltd, we are dedicated to maintaining a quality management system that is customer-focused and process-driven.
We are committed to continuously enhancing our effectiveness in line with our ISO 17015 accreditation and ISO 21501-4 calibration equipment
We are committed to delivering complete project management, validation services, and technical training to our clients. The team consists of Project Management Professionals (PMP), qualified engineers and System Integration Specialists with extensive experience in Life Sciences monitoring systems, software, and applications.
Link Lab’s wealth of experience with numerous validated systems is crucial to the success of every project, addressing both technical and logistical challenges. This accumulated expertise has enabled our team to operate efficiently and effectively, ensuring that we meet our customers’ requirements in terms of scope, schedule, and budget.
Our Quality Policy aims to not only meet but exceed the needs and expectations of our customers.
We align all personnel activities towards customer satisfaction by consistently enhancing the quality of our products and services.
